Informatics Institute faculty member Prof. Dr. Adem Tekin coauthored paper titled 'Crystal Structure Prediction and Dehydrogenation Mechanism of LiMg(BH
4)
3(NH
3)
2' has been published in '
The Journal of Physical Chemistry C'.
https://doi.org/10.1021/acs.jpcc.1c00127
Abstract:
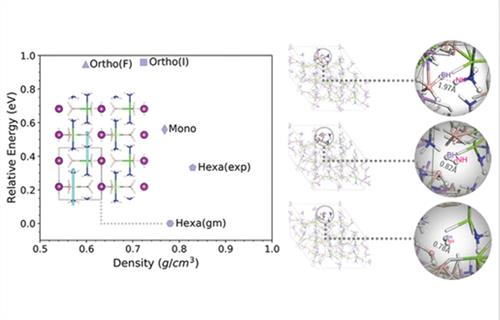
Dual-cation ammine metal borohydrides are favorable hydrogen storage materials due to their high gravimetric density and relatively low hydrogen release temperature. By combining the Fast and Flexible CrystAl Structure Predictor with density functional theory calculations and Car-Parrinello molecular dynamics, we studied the polymorphism, the lattice stability, and the decomposition mechanism of LiMg(BH4)3(NH3)2 in the temperature range 100–700 K. The onset of H2(g) formation is found at 400 K through the recombination of the hydrogen atoms from the bond cleavage of B–H and N–H in BH4 and NH3 groups. In addition to two hexagonal structures, of which one is the global minimum structure (
P63/
m)) and the other corresponds to the experimentally proposed room-temperature structure (
P63), a monoclinic (
Cc) structure and two orthorhombic structures (
Fmm2,
Ima2) are proposed as stable structures.